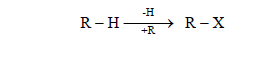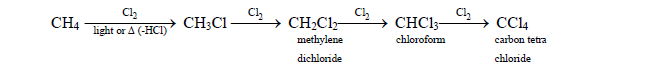HALO-ALKANES
Introduction
The halogen derivative of an alkane is called haloalkane.
Functional group = X (Cl, Br, I)
General formula = R-X ( CnH2n+1X )
Classification (on the basis of nature of carbon bonded with X)
i. Primary alkyl halide (1º)
ii. Secondary alkyl halide (2º)
iii. Tertiary alkyl halide (3º)
Nomenclature
Common name – named as Alkyl halide
IUPAC name – named as Halo alkane
Isomerism
Alkyl halide can exhibit the following types of isomerism.
Preparation
1. From alkane : (By halogenations)
Halogenation = Substitution of H by halogen atoms
The product is a mixture of mono and polysubstituted halides.
2. From alkene (Addition of HX)
Markonikov's ruleWhen an unsymmetrical reagent (HX) is added to an unsymmetrical alkene, the +ve part of the reagent (H) is added to that carbon of the double bond which already contains greater no. of H, and the –ve part (X) is added to that carbon of the double bond that contains lesser no. of hydrogen. This rule is called Markonikov's rule.
3. From alcohol
a. with HX
Alcohol reacts with HX in presence of dehydrating agents like anhydrous ZnCl2 or conc. H2SO4 to give alkyl halide.
b. With phosphorus halide & thionyl chloride
This method is preferred because the other products (HCl & SO2) are gaseous & escape out leaving behind pure haloalkane.
Physical Properties of Halo-alkanes
1. Physical state & odour
The lower members (eg – Bromo methane, chloroethane) are gases. Iodo ethane is a colorless liquid with having a sweet smell.
2. Boiling Point
- Haloalkanes have a higher Boiling point than corresponding alkanes.
-The boiling point decreases with branching.
- The order of boiling point is RF < RCl < RB < RI
3. Solubility
The alkyl halides are insoluble in water and are soluble in organic solvents.
Chemical Properties of Halo-alkanes
A. Nucleophilic substitution reaction
B. Elimination reaction
Dehydrohalogenation
Saytzeff's rule
In the elimination reaction, the preferred product (major product) is the alkene that has a greater no. of alkyl groups attached to the doubly bonded carbon atom.
C. Reaction with metals
(i) With Mg (Grignard reagent)
(ii) With sodium (Wurtz reaction)
iii) With Zn
D. Reduction