Thermodynamics Notes
Zeroth law of Thermodynamics:
When two bodies A and B are in thermal equilibrium with third body C then body A and B also must be in thermal equilibrium with each other.
First law of Thermodynamics
When heat energy is given to a system then some part of heat energy supplied is used to change the internal energy of the system and the rest of the energy is used to do external work.
Internal energy (U).
The sum of energy due to molecular motion (KE) and due to molecular configuration (PE) is called the internal energy of the gas.
:. Internal energy (U) = PE + KE
:. Internal energy (U) = PE + KE
For the ideal gas intermolecular force of attraction is neglected so PE = 0 so the internal energy of the ideal gas is KE which is only the function of temperature.
Work (W)
Work is said done only when the volume of gas changes. Work done by the gas is taken positively for expansion and work done on the gas is taken negatively for compression.
The area under the PV curve between the volume axis is equal to the work done. For the closed cycle, the area of the closed-loop gives the work done.
The area under the PV curve between the volume axis is equal to the work done. For the closed cycle, the area of the closed-loop gives the work done.
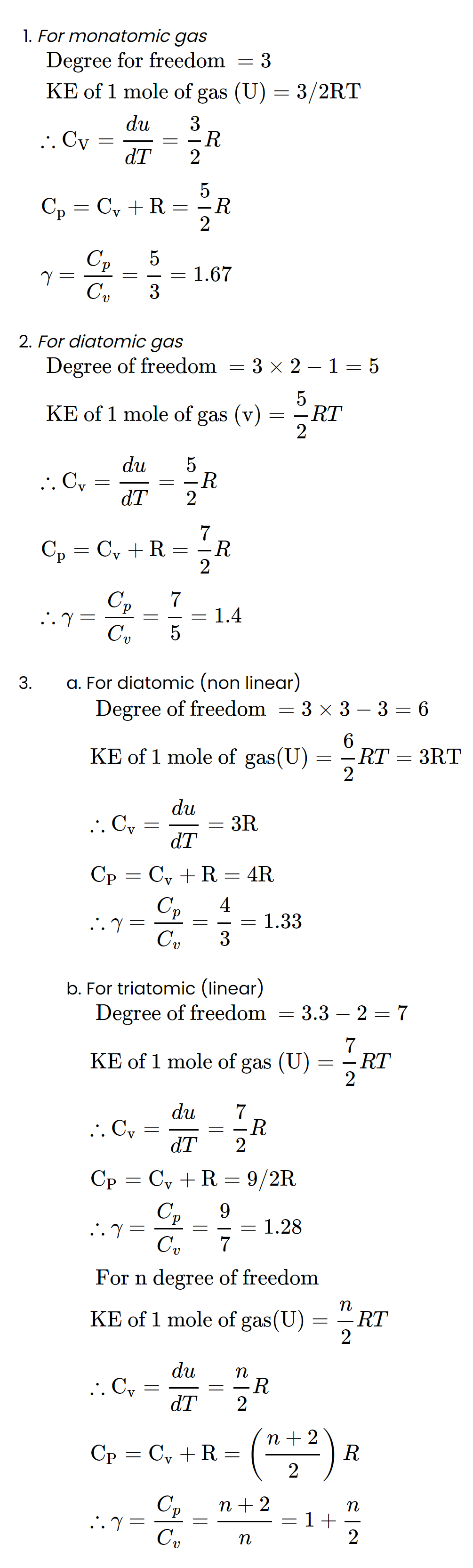
Heat capacities of gas
Heat required to raise the temperature of a certain amount of gas at constant pressure is always greater than the heat required to raise the same temperature at a constant volume so gas has two types of heat capacities.
1. Molar heat capacities
A. Molar heat capacity at constant pressure (Cp)
Heat is required to raise the temperature of one mole of gas through 10C
B. Molar heat capacity at constant volume (Cv)
Heat required to raise the temperature of one mole of gas through 10C
at constant volume. It's unit is Jmol-1K-1.
:. Heat required (dU) = nCvdT
C. Mayer's Formula
Cp - Cv = R
2. Specific heat capacities
A. Specific heat capacity at constant pressure (cp).
Heat required to raise the temperature of unit mass of gas through 10C at constant pressure. Its unit is Jkg-1K-1
Cp = M. cp
B. Specific heat capacity at constant volume (cv)
Heat required to rise the temperature of a unit mass of gas through 10C at constant volume. Its unit is Jkg-1K-1.
Heat required (du) = ncvdT
=> Cv = M.cv
Now, cp – cv = R/M = r